17-allylamino,17-demethoxygeldanamycin (17-AAG) is a novel anticancer drug that is a less toxic analogue of the geldanamycin which binds to Hsp90 and alters its function. HSP90 client proteins play important roles in the regulation of the cell cycle, cell growth, cell survival, apoptosis, and oncogenesis.
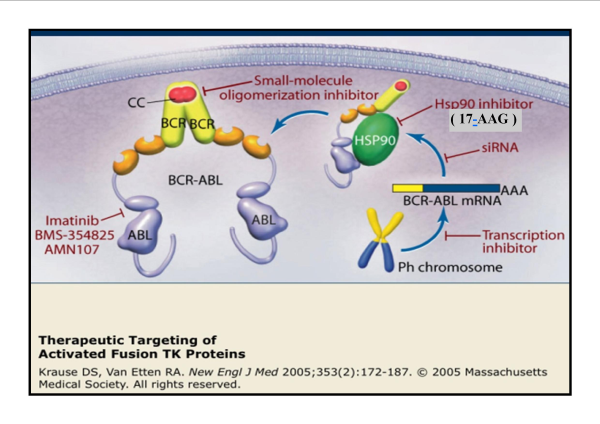
17-allylamino,17-demethoxygeldanamycin (17-AAG) is a novel anticancer drug that is a less toxic analog of the geldanamycin which binds to Hsp90 and alters its function. HSP90 client proteins play important roles in the regulation of the cell cycle, cell growth, cell survival, apoptosis, and oncogenesis. 17-AAG (Geldanamycin) binds into the ATP binding pocket in Hsp90 and induces the degradation of proteins that require this chaperone for conformational maturation. 17-AAG (Geldanamycin) inhibits Akt activation and expression in tumors and synergizes with a number of antitumor agents such as taxol, cisplatin, and UCN-014.
17-AAG is used to fight against prostate cancer, breast cancer, thyroid cancer, human uveal melanoma cell lines, proliferation of glioma cell lines and glioma stem cells, kidney tumors, and more tumor-related diseases due to its great procedure of inhibiting the proliferation of cells.
17-AAG HAS THE ABILITY TO
- inhibit the growth of both human glioma cell lines and glioma stem cells in vitro and is able to target the appropriate proteins within these cells
- inhibit the growth of intracranial tumors and can synergize with radiation both in tissue culture and in intracranial tumors
- degrade polyglutamine-expanded mutant androgen receptor
- reduce amounts of monomeric and aggregated mutant androgen receptor
- disrupt the Hsp90-client protein complexes and lead to the degradation of the client proteins
- regulate oncoproteins by binding specifically to heat shock protein 90 (HSP90)
- contribute to the destruction of proteins in cells that may play a role in causing cancer and spurring tumor growth
- ameliorate motor impairments in the SBMA transgenic mouse model without detectable toxicity, by reducing amounts of monomeric and aggregated mutant androgen receptor
- ameliorate polyglutamine-mediated motor neuron degeneration
TOXICITY OF 17-AAG IN ANIMALS
Single-dose range finding, multiple-dose range finding, 5-day daily dose and multiple-dose/dimethyl sulfoxide (DMSO) formulation toxicity studies have been conducted in rats. Additionally, single-dose range-finding/micro dispersed formulation and multiple-dose range finding reconstituted lyophilized formulation, and 5-day daily dose with micro dispersed and DMSO-formulated 17-AAG have been conducted. In those studies, the following trends were noted.- Doses of GA exceeding 5mg/kg in rats were generally toxic, leading to death.
- A single dose of micro dispersed 17-AAG could be given in doses up to 25 mg/kg in both rats and dogs; the maximum tolerated dose (MTD) when given daily for 5 days was 25 mg/kg/day for rats and 7.5 mg/kg/day for dogs.
- Lyophilized 17-AAG was tolerated in rats at doses up to 30 mg/kg when given daily or twice daily and at 10 mg/kg/day in dogs.
- Hepatoxicity, renal failure, and gastrointestinal (mainly emesis and diarrhea) toxicities were the dose-limiting toxicities (DLTs) in both species. Dogs also experienced gallbladder toxicities.

The molecular target of 17-AAG is the chaperone HSP90, which, depending on its conformation, recruits various cochaperones to assemble two distinct multi chaperone complexes. One complex (I) favors the proteasome-dependent degradation of HSP90 client proteins, whereas the other complex stabilizes HSP90 client proteins. 17-AAG binding to HSP90 removes the conformational flexibility of the chaperone, resulting in accumulation of the proteasome-targeting HSP90-based multi chaperone complex with concomitant loss of the client protein stabilizing HSP90 complex. The result is coordinated degradation of the HSP90 clients HER-2, Akt, Raf-1, and androgen receptor.

